06/03/2022
Challenges in the Development of a Method for the Detection of Anti-PEGylated-aptamer Antibodies
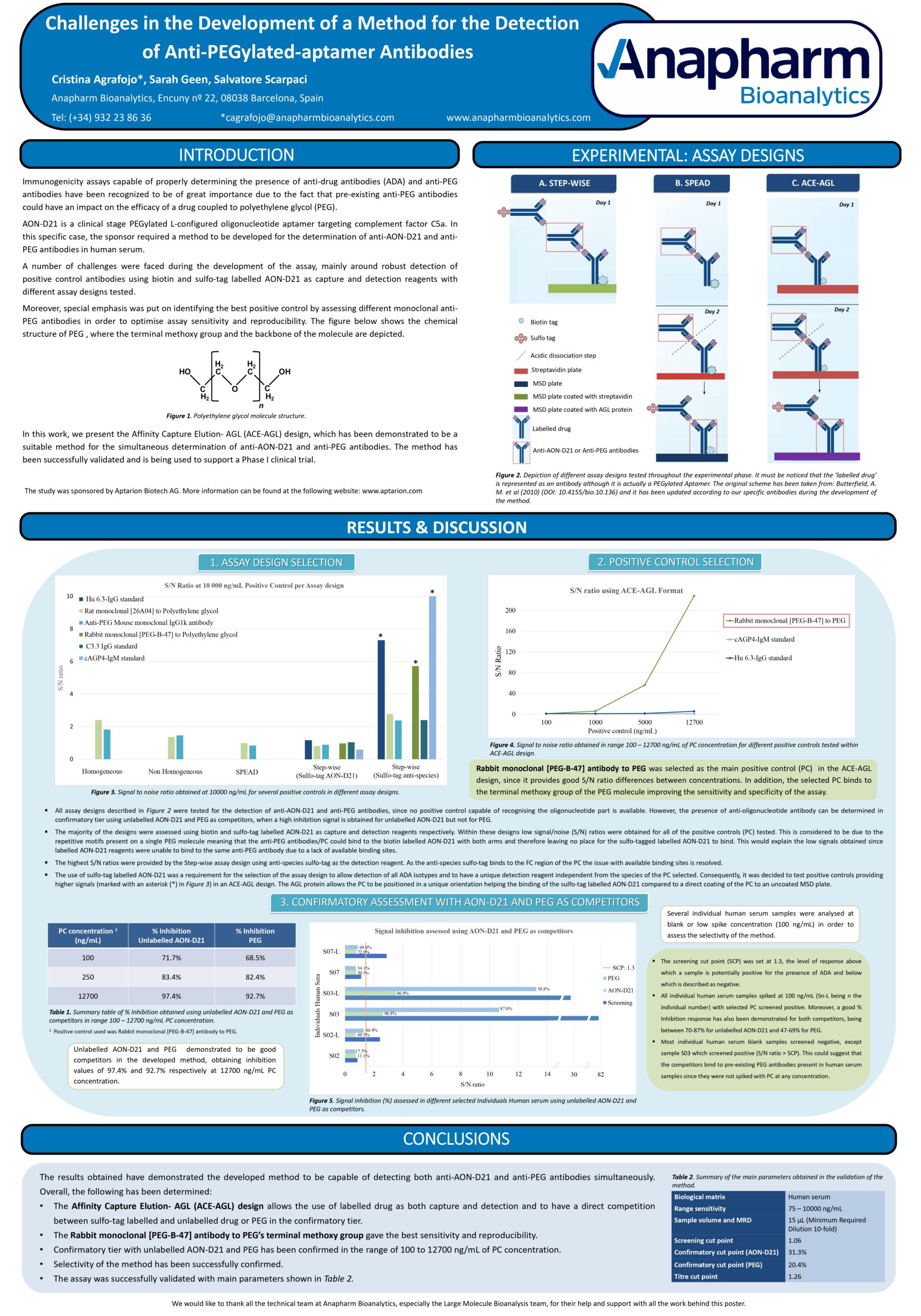
Immunogenicity assays capable of properly determining the presence of anti-drug antibodies (ADA) and anti-PEG antibodies have been recognized of great importance due to the fact that pre-existing anti-PEG antibodies could have an impact on the efficacy of a drug coupled to polyethylene glycol (PEG).
AON-D21 is a clinical stage PEGylated L-configured oligonucleotide aptamer targeting complement factor C5a. In this study, the sponsor required a method to be developed for the determination of anti-AON-D21 and anti-PEG antibodies in human serum.
In the work we present to the 8th EBF Young Scientist Symposium, a number of challenges were faced during the development of the assay mainly around robust detection of positive control antibodies by using biotin and sulfo-tag labelled AON-D21 as capture and detection reagents. Several methods were evaluated with the most optimal assay design being the Affinity Capture Elution- AGL (ACE-AGL) format, which captures the ADA binding to immobilized biotin-AON-D21, followed by an acid elution step of ADA and finally the re-capture of these on a MSD plate coated with protein AGL. The ADA are then detected using sulfo-tag labelled AON-D21. In confirmatory assays, this is competed by unlabelled AON-D21 or PEG alone to identify the ADA binding site.
Special emphasis was put in identifying the best positive control by assessing different monoclonal anti-PEG antibodies in order to optimise assay sensitivity and reproducibility.
Finally, a suitable method capable of detecting both anti-PEG and anti-AON-D21 antibodies simultaneously was developed. The method has been validated and is being used to support a Phase I clinical trial in humans
You may find below the poster for your ready reference:

MORE NEWS
Peptides Series: Understanding Therapeutic Peptides
Therapeutic peptides are transforming modern medicine thanks to their unique advantages over traditional drug therapies. Their molecular structure and biological properties allow them to achieve effects like natural molecules in the body, enabling targeted action with fewer risks of off-target effects.
The visit of the Mayor of Barcelona, Jaume Collboni, to Anapharm Bioanalytics
Last Friday, January 10th 2025, we had the honor of welcoming the Mayor of Barcelona, Jaume Collboni, to our facilities. His visit was a special opportunity for us to showcase the work we do at Anapharm Bioanalytics and to reaffirm our commitment to innovation in the field of bioanalysis.
Overcoming Matrix Effects in the Analysis of Lipophilic Compounds
At the 10th EBF Young Scientist Symposium, we highlighted the complexities of analysing Fucoxanthin derivatives and demonstrated how innovative approaches, such as 2D Chromatography, can overcome these obstacles.

